Resources
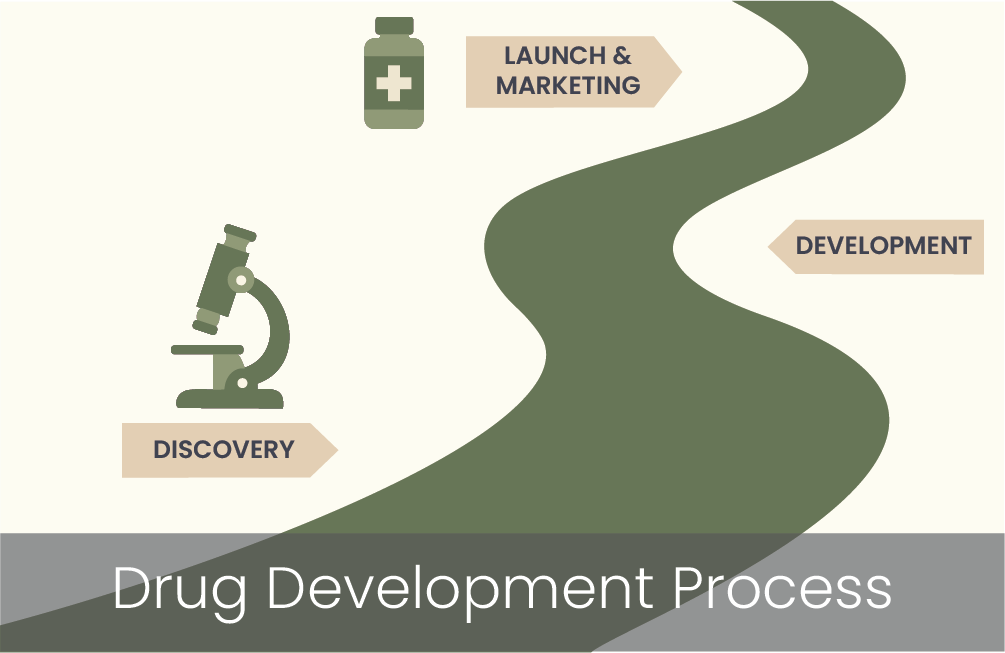
Drug Development Process
The drug development process is a systematic, multi-stage journey to bring a new therapy from discovery to development to market. It typically spans 10–15 years and involves rigorous testing to ensure safety, efficacy, and quality.
Clinicaltrial.gov
ClinicalTrials.gov is a publicly accessible online database that provides detailed information about clinical studies conducted worldwide. It serves as a central resource for patients, researchers, and healthcare professionals to learn about ongoing and completed clinical trials.