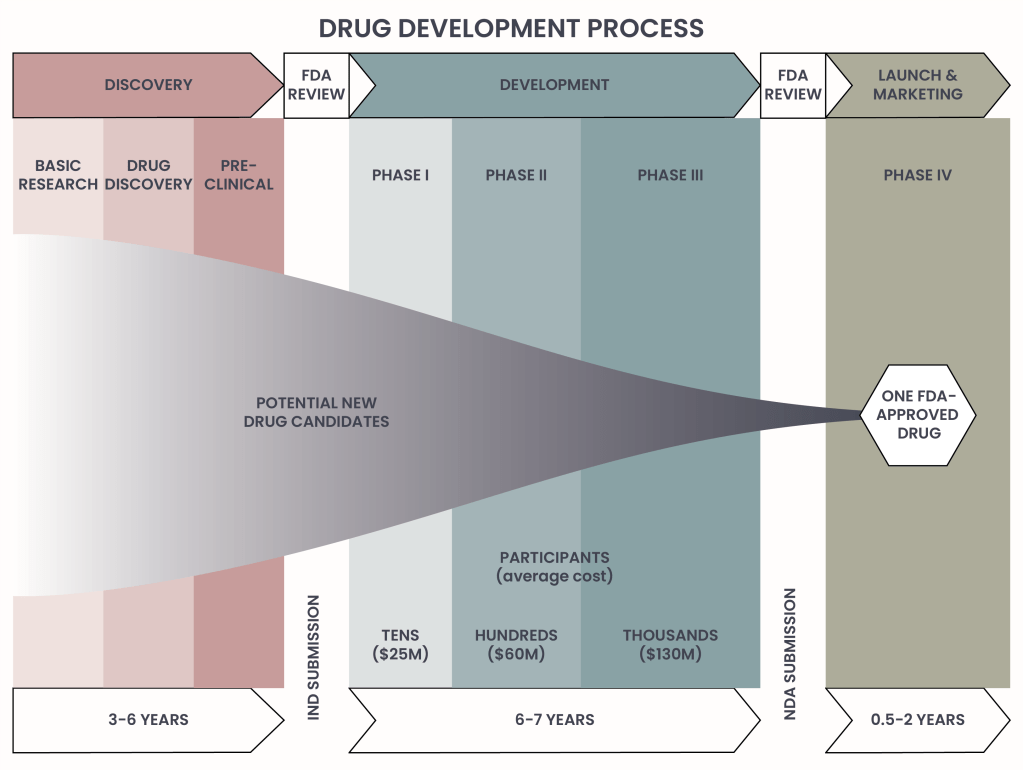
Drug development process
The journey of developing a new drug is complex, rigorous, and often spans over a decade. Each step in the process is designed to ensure that therapies brought to market are safe, effective, and capable of improving patient outcomes. From the initial exploration of disease mechanisms to long-term monitoring of approved treatments, every phase plays a critical role in transforming scientific discoveries into life-changing medicines. Below, we break down the drug development process, highlighting the key milestones that turn innovative ideas into approved therapies.
DISCOVERY
This foundational phase involves exploring fundamental biological mechanisms. Scientists investigate the molecular pathways, genetic factors, and cellular behaviors to understand diseases better. Insights gained during basic research lay the groundwork for identifying potential drug targets.
In this phase, researchers identify compounds or molecules that could interact with the biological targets identified during basic research. High-throughput screening, computational modeling, and medicinal chemistry are used to find and test promising candidates. These “hits” are optimized to improve their efficacy and safety, resulting in lead compounds for further testing.
Before testing in humans, potential drugs undergo rigorous evaluation in the lab and in animal models. Pre-clinical studies assess the compound’s safety, toxicity, pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted), and pharmacodynamics (how the drug affects the body). Success at this stage is necessary to advance to clinical trials.
To begin human testing, researchers submit an Investigational New Drug (IND) application to regulatory authorities (like the FDA). The application includes pre-clinical data, manufacturing details, and the proposed clinical trial plan. Approval ensures the drug candidate meets safety standards for testing in humans.
DEVELOPMENT
These early trials focus on safety. A small group of healthy volunteers or patients (20-50 participants) receives the drug to determine the safe dosage range, pharmacokinetics, and potential side effects. Phase I trials often involve escalating doses to identify the maximum tolerated dose. The average cost for a phase I clinical trial is $25M.
In this phase, the drug’s efficacy is evaluated in a larger group of patients (approximately 100 participants) with the targeted condition. Researchers also gather more data on safety and dosing. Phase II trials are critical for determining whether the drug demonstrates enough benefit to justify larger-scale testing. The average cost for a phase I clinical trial is $60M.
These large-scale studies assess the drug’s effectiveness compared to standard treatments or placebo across diverse populations (100-1000 participants). Phase III trials provide comprehensive data on safety, efficacy, and overall benefit-risk profile. Results from this phase often form the basis for regulatory approval. The average cost for a phase I clinical trial is $130M.
With successful Phase III results, the sponsor submits an New Drug Application (NDA) to regulatory authorities like the FDA, seeking approval to market the drug. The NDA includes all pre-clinical and clinical data, as well as details about manufacturing and labeling. Regulatory review ensures the drug meets safety, efficacy, and quality standards.
LAUNCH & MARKETING
After regulatory approval, Phase IV trials monitor the drug’s real-world use to detect rare side effects, long-term outcomes, and effectiveness in broader populations. These studies ensure continued safety and may explore new indications or patient groups.