Clinicaltrials.gov
ClinicalTrials.gov is a comprehensive database of privately and publicly funded clinical studies conducted around the world. The website, maintained by the U.S. National Library of Medicine, allows users to search for information on ongoing and completed clinical trials for a wide range of conditions, interventions, and treatments. On ClinicalTrials.gov, you can explore detailed study descriptions, including objectives, eligibility criteria, study locations, and results. It serves as a valuable resource for patients seeking clinical trial opportunities, healthcare professionals looking for the latest research, and researchers or sponsors needing to register and share study details.
Here, you’ll find a brief overview of how to use Clincaltrial.gov to search for past and ongoing clinical trials. Your search can be filtered by using certain factors:
- Condition/disease,
- Number, name, industry sponsor, or lead researcher of the clinical trial,
- Intervention/treatment,
- Location,
- Study status,
- More.

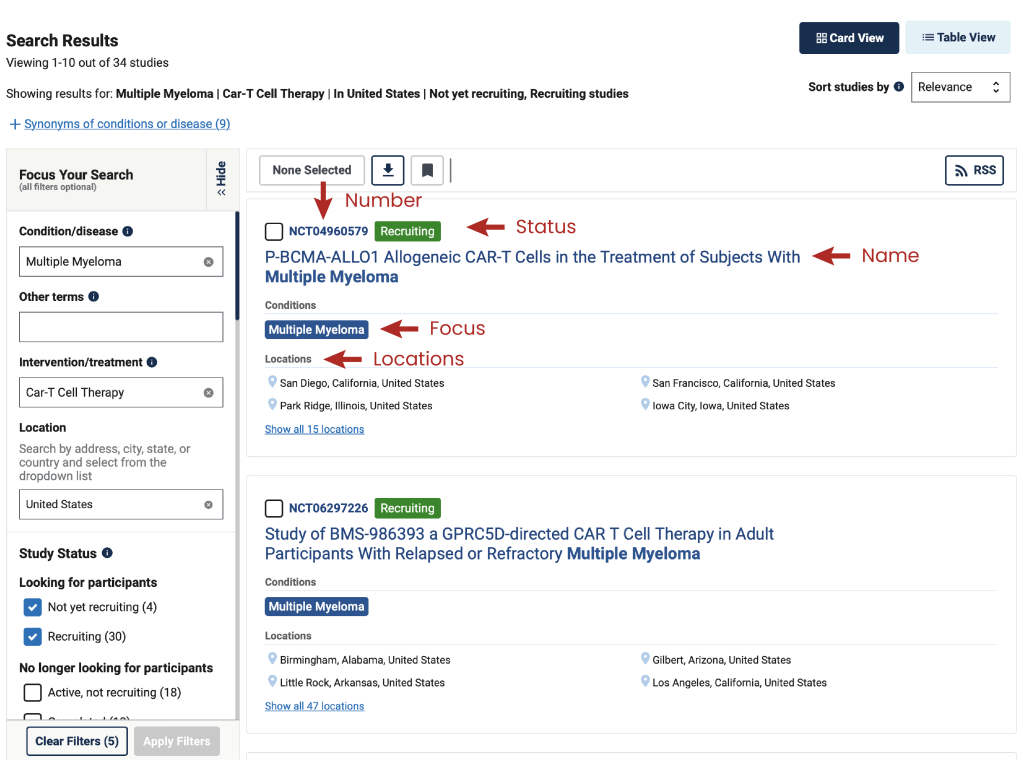
Clinicaltrial.gov will provide you with results based on the criteria used for the search. In the sidebar on the right, you are able to adjust the current search or start a new one. Each result of the search gives you first information about study name, status, number, focus and locations.
Each study details page contains the following information:
- Brief study overview and and detailed description,
- Contacts and locations,
- Participation criteria,
- Study plan,
- Collaborators and investigators,
- Publications,
- Study record dates,
- More.
In addition, you will find key information about the study such as the timeline for recruitment, how many patients will be recruited, and the study type. Studies listed in Clinicaltrial.gov are maintained by the study sponsor and may not always be kept up to date. If you would like to learn more about the study or are interested in participating in the clinical trial you should reach out to the contact provided in the contact section. To determine if a patient is eligibile to participate in the clincal trial, patient must have the listed characteristics in the inclusion criteria and may be exluded based on the exclusion criteria.

Once a study obtains results it will be shared in the results tab.
ClinicalTrials.gov is not the only resource to find and educate yourself on past, present, and future clinical trials. There are many websites available for patients to find the best trial for them. Here are other alternatives provided by national clinical trial websites.

