Hearing the words “You have cancer” is life shattering, but for many, hearing “You’re cancer-free” doesn’t always bring the peace and relief one might expect. Living a life after a cancer treatment often comes with a new set of psychological challenges. Many patients quietly wonder, “Am I really cancer-free, or could there still be cancer cells hiding?” or “What if metastases are growing unnoticed until it’s too late?”. These lingering fears can cloud the ability to fully embrace life after treatment. This is where the science of Minimal Residual Disease (MRD) comes in.
What is Minimal Residual Disease (MRD)?
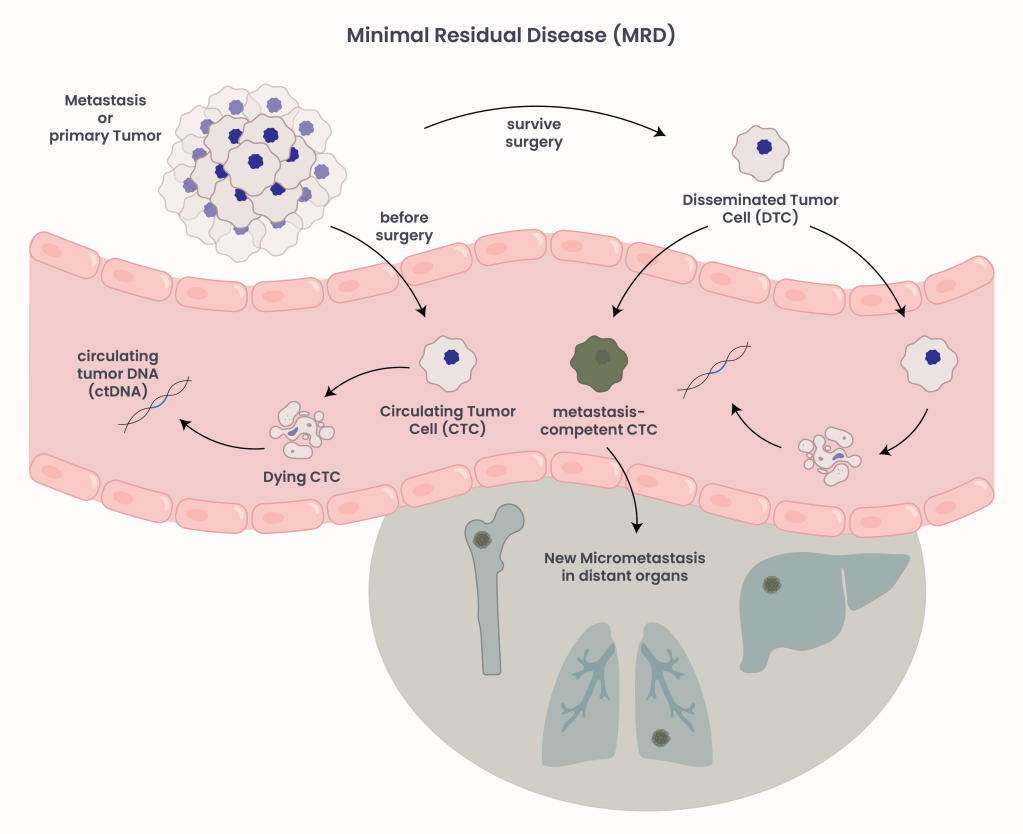
Figure 1. Sources of tumor DNA in the blood.
MRD refers to the small number of cancer cells that may remain in the body after treatment, even when imaging shows no signs of disease. These cells, called disseminated tumor cells (DTCs), detached from the primary tumor or existing metastases, survive treatment, and spread throughout the body. Residual cells can eventually lead to disease recurrence or metastases, which is why detecting MRD is crucial1. Traditional imaging methods are effective at detecting tumors of a certain size (computer tomography can detect tumors as small as 2-3 mm), but they lack the sensitivity to identify single cancer cells that may be circulating in the bloodstream. Once DTC enter the bloodstream they face two possible outcomes (Figure 1):
- They die, releasing fragments of their DNA known as circulating tumor DNA (ctDNA) into the blood.
- They survive, eventually gaining the ability to establish new metastatic tumors in distant organs.
MRD testing focuses on detecting these tiny traces of ctDNA through a simple blood sample. The presence of ctDNA suggests that tumor cells persist, and increases risk of cancer recurrence after a cure (surgery or other treatments). Even if patients with the same tumor burden present with similar ctDNA concentrations, their course of disease might vary. Metastasis can differ between individuals depending on factors like the cells and environment around the tumor, called tumor microenvironment, or unique mutations present. Tumor cells can shed from the primary tumor even before surgical resection, called circulating tumor cells (CTCs). Likewise, upon cell death, CTCs release ctDNA into the bloodstream. However, because ctDNA is only stable for a short duration (typically minutes) it is unlikely to be the source of ctDNA detected after surgery.
Why is MRD Testing Important?
MRD testing has the potential to answer some of the most pressing questions patients and clinicians face after treatment, which helps improve clinical decision making:
- Should I receive additional therapy?
MRD can help decide if adjuvant chemotherapy is needed to eliminate residual cancer cells and reduce the risk of relapse. Likewise, identifying patients without residual cancer cells can spare them from adjuvant chemotherapy and its side effects. - Can recurrence be detected earlier?
MRD testing may identify cancer recurrence earlier than standard imaging techniques, which improves patient outcome. - Who is at higher risk?
It can help identify patients with a higher risk of progression, guiding decisions around more frequent monitoring or additional treatments.
Promising MRD tests: A Closer Look
The following section describes three of the most promising tests currently available. Although none of these tests have received FDA approval, they are approved under the Clinical Laboratory Improvement Amendments (CLIA), which ensures the quality and accuracy of laboratory testing performed on human samples in the U.S. This CLIA certification allows these tests to be used in clinical trials and routine clinical testing; however, their safety and efficacy must still be demonstrated to obtain FDA approval. To emphasize their potential utility in clinical decision-making, the results from the most recent study for each test will be summarized.
Signatera2 by Natera
Signatera is a personalized test designed to detect ctDNA in cancer patients. Each test is customized to the individual patient based on a comparison of the DNA profile of normal tissue versus the tumor tissue obtained during the surgery, which makes the test highly sensitive. Unfortunately, adequate tumor tissue, either in quantity or quality, is not always available for every patient, limiting the applicability of this test. Up to today, oncologists have ordered the test for >250,000 patients covered by medicare for stage II-IV colorectal, stage II-IV breast, stage II-III non-small cell lung, stage II-IV ovarian, and muscle invasive bladder cancer patients.
- CIRCULATE-Japan GALAXY study3 is an observational study that enrolled 2,240 patients with resectable stage II–IV colon cancer. MRD test was performed 2-10 weeks after surgery with curative intent and each patient was followed for up to 49 months. The observation showed that ctDNA-positive patients were more likely to recur and had a reduced overall survival. Additionally, poor outcomes in MRD-positive patients could be improved with adjuvant therapy, while adjuvant therapy in MRD-negative patients had no improving effect. This suggests that the Signatera MRD test may be able to predict when further treatment will be effective, and mitigate unnecessary procedures.
Guardant Reveal4 by Guardant Health
Guardant Reveal is a test designed to detect ctDNA in stage II or III colorectal, breast, and lung cancer patients. This test levarages epigenetic modifications to distinguish between tumorous and normal DNA. Medicare covers the costs for patients with stage II or III colorectal cancer whose testing is initiated within 3 months following curative intent therapy.
- COnquer Solid Malignancies by blOod study5 (COSMO) is a screening of ctDNA in 342 patients with resected stage II or higher colorectal cancer. Results showed that ctDNA-positive patients were more likely to recur. On average, ctDNA was detected 5.3 months before recurrence was clinically confirmed by imaging.
CLARITY6 by Foresight Diagnostics
CLARITY is a test designed to detect ctDNA in non-small cell lung cancer (NSCLC) and breast cancer patients. Unlike Signatera, CLARITY does not require tumor tissue. Instead, it is designed to detect multiple known cancer-associated mutations occurring simultaneously on the same small fragment of circulating tumor DNA (ctDNA), allowing it to differentiate cancer-derived DNA from normal DNA. This unique approach enhances the sensitivity of ctDNA detection.
- TRAcking Non-small cell lung Cancer Evolution Through Therapy (Rx) study7 (TRACERx, NCT018886018) enrolls 46 patients with resectable NSCLC measuring ctDNA presence in the blood. Results showed that ctDNA-positive patients after surgery were more likely to recur, but adjuvant therapy could improve patient outcome.
By providing a clearer picture of residual tumor cells after treatment, MRD testing can empower patients and healthcare teams to make more informed decisions. It also has the potential to reduce the emotional burden of uncertainty, allowing patients to focus on recovery and living life to the fullest.
- Pantel K, Alix-Panabières C. Minimal residual disease as a target for liquid biopsy in patients with solid tumours. Nat Rev Clin Oncol. 2025;22(1):65-77. doi:10.1038/s41571-024-00967-y. ↩︎
- Natera. (n.d.), Setting the standard for MRD testing. natera.com. Retrieved March 29 2025 from https://www.natera.com/info/personalized-mrd-test/. ↩︎
- Nakamura, Y., Watanabe, J., Akazawa, N. et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat Med 30, 3272–3283 (2024). https://doi.org/10.1038/s41591-024-03254-6. ↩︎
- Guardant Health. (n.d.), Guardant Reveal™—fast, tissue-free minimal residual disease (MRD) detection. guardanthealth.com. Retrieved March 29 2025 from https://www.guardantcomplete.com/products/guardant-reveal/. ↩︎
- Nakamura Y, Tsukada Y, Matsuhashi N, et al. Colorectal Cancer Recurrence Prediction Using a Tissue-Free Epigenomic Minimal Residual Disease Assay. Clin Cancer Res. 2024;30(19):4377-4387. doi:10.1158/1078-0432.CCR-24-1651. ↩︎
- Foresight Diagnostics. (n.d.), Foresight CLARITY™ MRD Platform. foresight-dx.com. Retrieved March 29 2025 from https://foresight-dx.com/foresight-products-services/foresight-clarity/#solid-tumors. ↩︎
- Isbell JM, Goldstein JS, Hamilton EG, et al. Ultrasensitive circulating tumor DNA (ctDNA) minimal residual disease (MRD) detection in early stage non-small cell lung cancer (NSCLC). Poster presented at: American Society of Clinical Oncology (ASCO); 2024. (See poster). ↩︎
- National Library of Medicine. (n.d.), TRAcking Non-small Cell Lung Cancer Evolution Through Therapy (Rx) (TRACERx). clinicaltrial.gov. Retrieved March 29 2025 from https://clinicaltrials.gov/study/NCT01888601/. ↩︎
© 2025 WithinOncology. All rights reserved.
This article, including all text, tables, and figures, is the intellectual property of WithinOncology and its contributors. Unauthorized reproduction, distribution, or use of any content without explicit written permission is strictly prohibited. For inquiries, please contact us via the contact form.


Leave a comment